All-in-one molecular diagnosis cartridges and analysis equipment
technology allowing for quick on-site diagnosis of infectious disease
(swine flu, MERS, STD) by a semi-professional
Client / Market
- In Vitro diagnostic, molecular diagnosis market
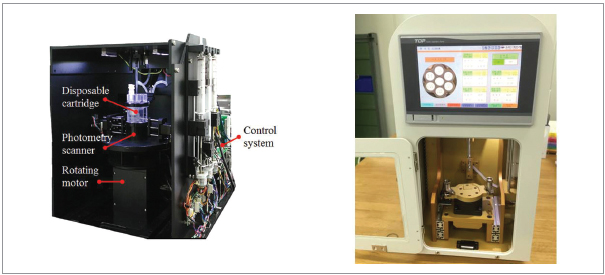
Necessity of this Technology
- The occasions calling for quick diagnosis due to the emergence of new virus
diseases are increasing, and the demand for the DNA test for disease prevention
has increased.
- The DNA test for detecting virus mostly uses the PCR method or isothermal
amplification, so the test is conducted with an electrophoresis tool or a real-time
PCR machine. However, out of the necessity at the field, a simpler, automated DNA
test is demanded.
- A lot of time is consumed for pre-treatment of a specimen, mixing of a specimen
and a reagent, and the residue treatment. And the existing specimen pre-treatment
device has a complex structure with high production cost and consumable
expenses. While treating a large amount of specimens at once, the specimen could
possibly be contaminated.
Technical Differentiation
- It is mechanically simple and effectively handles the specimen pre-treatment.
- It automatizes the entire process – nucleic acid extraction, amplification and
detection
- Nucleic acid extraction is easy. (30 minutes. 60 to 90 minutes for testing)
- It includes the chambers that store reagents to be mixed with the specimen, and the
reagents are connected to the specimen pre-treatment device, where specimens
are discharged from each chamber in order according to the pre-treatment
process order. This simplifies the pre-treatment device and integrates the nucleic
acid amplification and detection device, which the nucleic acids extracted from the
specimen flow in.
| Process Order |
Component/Process |
Additional Explanation |
| Nucleic acid extraction |
Modify disposable cartridge and joint design
Optimize cartridge driving module |
Nucleic acid extraction time < 30 min.
*May differ depending on the test subject and item |
| Nucleic acid amplification |
Precise temperature control (heating/cooling) module
Prototype modification |
Nucleic acid amplification, detection time < 30 min
*May differ depending on the test subject and item |
| Nucleic acid detection |
| Diagnosis analysis |
Integrated system for nucleic acid extraction, amplification,
and detection and control and analysis assessment |
Target test time < 40 to 60 min |
Excellence of Technology
- Prototype process chart
- Through the internal research project and the government-entrusted assignment,
the prototype for the fully automatic nucleic acid extraction with the all-in-one
cartridge and the single channel isothermal (61 degree) amplification and detection
was produced and its performance was verified.
- The basic system for multiplexing PCR through multichannel optical modules was
completed.
CURRENT INTELLECTUAL PROPERTY RIGHT STATUS
PATENT
- Sample Preparation Device (KR1244467)
- CN201110428842.3)
- Device for Automatically Analyzing Nucleic Acid and Opening and Closing Device Thereof (KR1487537)
- A Cartridge for Automatically Analyzing a Nucleic Acid (KR1512161)
- Cartridge for Sample Preparation (KR1630784)
- Cartridge for Sample Preparation and Collected Acid Analysis (KR1703992)
|





